A breakthrough in our understanding of phytase enzyme mode of action and the associated matrix values in feed formulation has been provided in a recent paper published by Tran et al. in the January 2011 edition of Analytical Biochemistry entitled ‘A simple and fast kinetic assay for phytases using phytic acid–protein complex as substrate’.
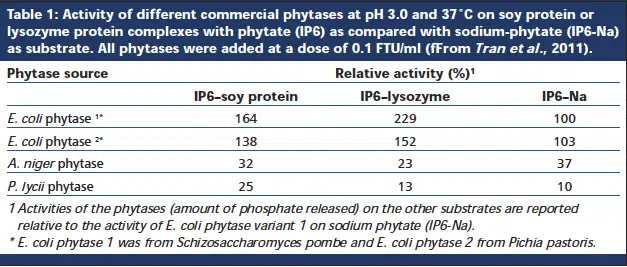
To fully understand the research results shown in Table 1 it is important to know that while sodium phytate is used as the substrate to determine phytase activity in-vitro, in nature and also in the acid part of the digestive tract of poultry (proventriculus and gizzard) and swine (stomach), phytic acid does not occur in its free acid or sodium salt form, but rather in association with proteins. The reason for the association of phytate and protein in the gut is that most proteins of plant origin, such as those derived from maize, soybean, sunflower and rapeseed (canola) meal, have their iso-electric points (pI values) in the acidic range (pH 4.5). Hence, when the pH in the digestive tract drops below the iso-electric point of the protein, the protein is now positively charged, which allows the negatively charged phytic acid molecule to bind to it, thereby altering the proteins iso-electric point and rendering the protein insoluble (Reddy et al., 1989; Konietzny and Greiner, 2003). This formation of insoluble protein-phytate complexes in the acid part of the digestive tract can have important nutritional consequences due to a decreased accessibility to pepsin proteases resulting in increased pepsin secretion, greater endogenous losses and inefficient protein digestion, as indicated by a reduced ileal amino acid digestibility from phytate. (Vaintraub and Bulmaga, 1991, Konietzny and Greiner, 2003, Kies et al., 2006, Cowieson et al., 2008).
Of further importance are new data that have shown the formation of insoluble protein-phytate complexes to be proportional to the amount of phytate present. This is shown in Figure 1 where the presence of insoluble protein-phytate complexes from either soy protein or casein, measured by absorbance at 600 nm, increased almost linearly as the phytate concentration increased. Stated another way, the anti-nutritional effect of phytate in the acid stomach of the animal will be proportional to the concentration of undigested phytate (IP6) present in the acid stomach and is further supported by previous work by Cowieson et al. (2009), who demonstrated a step-wise increase in endogenous amino acid losses at the terminal ileum as dietary phytate levels increased. Converse to the negative effects of phytate on energy and amino acid digestibility, nutritional advantages of phytase enzymes will depend primarily on the ability of the source of phytase to rapidly hydrolyze phytate-protein complexes at a low pH (gizzard and provetriculus region), thereby reducing their anti-nutritional effects and resulting in net improvements in energy and protein
utilization.
Peter Plumstead is the Technical director at Chemuniqué